Written By Lisa Murimi |
The United Kingdom’s state-run National Health Service is poised to revolutionize cancer treatment by becoming the pioneer in offering innovative injection therapy to cancer patients.
This breakthrough could potentially slash treatment durations by up to 75%. The Medicines and Healthcare Products Regulatory Agency (MHRA) has granted approval for this game-changing approach.
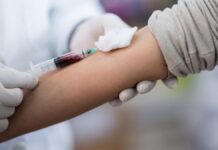
NHS England announced on Tuesday that eligible patients undergoing cancer treatment will receive an “under the skin” injection of atezolizumab, a cutting-edge immunotherapy. This remarkable advancement is set to not only accelerate treatment timelines but also create more availability for cancer care teams to attend to a larger number of patients.
Dr. Alexander Martin, a consultant oncologist at West Suffolk NHS Foundation Trust, emphasized the significance of this milestone. He stated,
“This approval will not only allow us to deliver convenient and faster care for our patients but will enable our teams to treat more patients throughout the day.” The potential of this innovation to enhance both patient experience and the overall efficiency of cancer care delivery cannot be understated.
As the UK’s national health service leads the charge in this groundbreaking endeavour, the world watches with anticipation, recognizing that this could potentially set a new standard for cancer treatment approaches. This development not only holds promise for the hundreds of patients in England but also offers hope to countless individuals battling cancer worldwide.