By Faith Mwende
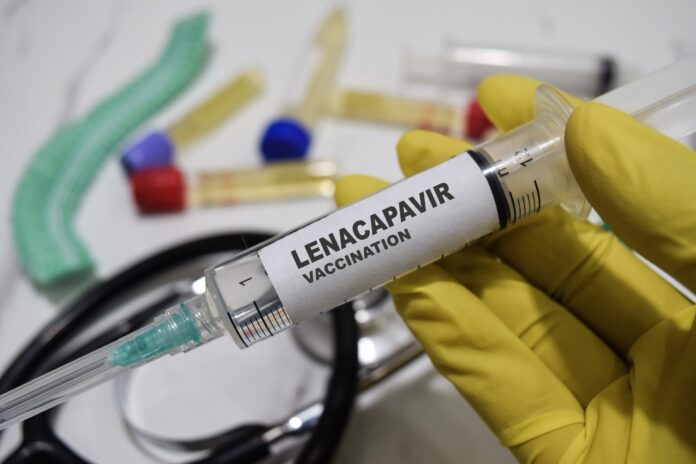
The South African Health Products Regulatory Authority (SAHPRA) has officially approved Lenacapavir, a long-acting antiviral drug designed to prevent HIV-1 infection in adults and adolescents weighing at least 35 kilograms.
Lenacapavir, developed by Gilead Sciences, is recommended for pre-exposure prophylaxis (PrEP), a preventive treatment for individuals at high risk of contracting HIV.
The drug, when used alongside safer sex practices such as condom use, significantly reduces the risk of HIV transmission.
The approval follows an application submitted by Gilead in March 2025 and a joint review process under the European Medicines for All (EU-M4all) initiative.
This collaborative framework allows the European Medicines Agency (EMA) and participating regulators to evaluate critical medicines intended for use outside the European Union, helping strengthen regulatory systems and speed up access to essential treatments.
Lenacapavir is administered as a subcutaneous injection every six months, following an initial dose that combines an injection and two oral tablets (taken on days 1 and 2). It is the first HIV prevention drug requiring only biannual dosing, offering a major step forward in simplifying HIV prevention and improving adherence.
SAHPRA CEO Dr. Boitumelo Semete-Makokotlela hailed the approval as a milestone in the country’s ongoing fight against HIV.
“The registration of Lenacapavir is a game-changer, given the high HIV prevalence in South Africa. This product represents the most effective HIV prevention measure developed to date,” she said.