The World Health Organization (WHO) today listed the Comirnaty COVID-19 mRNA vaccine for emergency use, making the Pfizer/BioNTech vaccine the first to receive emergency validation from WHO since the outbreak began a year ago.
The WHO’s Emergency Use Listing (EUL) opens the door for countries to expedite their own regulatory approval processes to import and administer the vaccine. It also enables UNICEF and the Pan-American Health Organization to procure the vaccine for distribution to countries in need.
“This is a very positive step towards ensuring global access to COVID-19 vaccines. But I want to emphasize the need for an even greater global effort to achieve enough vaccine supply to meet the needs of priority populations everywhere,” said Dr Mariângela Simão, WHO Assistant-Director General for Access to Medicines and Health Products.

Regulatory experts convened by WHO from around the world and WHO’s own teams reviewed the data on the Pfizer/BioNTech vaccine’s safety, efficacy and quality as part of a risk-versus-benefit analysis.
The review found that the vaccine met the must-have criteria for safety and efficacy set out by WHO, and that the benefits of using the vaccine to address COVID-19 offset potential risks.
The vaccine is also under policy review. WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) will convene on 5 January 2021, to formulate vaccine-specific policies and recommendations for this product’s use in populations, drawing from the SAGE population prioritization recommendations for COVID-19 vaccines in general, issued in September 2020.
The Comirnaty vaccine requires storage using an ultra-cold chain; it needs to be stored at -60°C to -90°C degrees. This requirement makes the vaccine more challenging to deploy in settings where ultra-cold chain equipment may not be available or reliably accessible.
For that reason, WHO is working to support countries in assessing their delivery plans and preparing for use where possible.
How does the emergency use listing work?
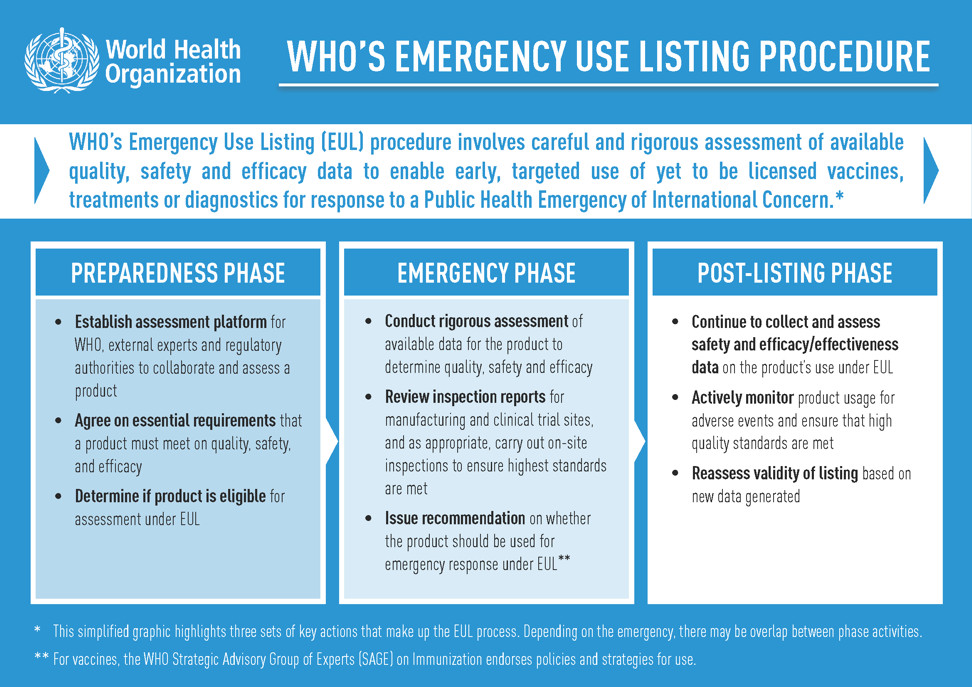
The emergency use listing (EUL) procedure assesses the suitability of novel health products during public health emergencies.
The objective is to make medicines, vaccines and diagnostics available as rapidly as possible to address the emergency while adhering to stringent criteria of safety, efficacy and quality.
The assessment weighs the threat posed by the emergency as well as the benefit that would accrue from the use of the product against any potential risks.
The EUL pathway involves a rigorous assessment of late phase II and phase III clinical trial data as well as substantial additional data on safety, efficacy, quality and a risk management plan.
Who Participates in reviewing EUL?
These data are reviewed by independent experts and WHO teams who consider the current body of evidence on the vaccine under consideration, the plans for monitoring its use, and plans for further studies.

Experts from individual national authorities are invited to participate in the EUL review. Once a vaccine has been listed for WHO emergency use, WHO engages its regional regulatory networks and partners to inform national health authorities on the vaccine and its anticipated benefits based on data from clinical studies to date.
As part of the EUL process, the company producing the vaccine must commit to continuing to generate data to enable full licensure and WHO prequalification of the vaccine. The WHO prequalification process will assess additional clinical data generated from vaccine trials and deployment on a rolling basis to ensure the vaccine meets the necessary standards of quality, safety and efficacy for broader availability.